A new scientific approach
> Read on to find out more.
Three core concepts

Phylotoxicology

Variation in
Susceptibility

Embedded
Translation
Objectives
- Stakeholder Integration, embedding the Stakeholder Advisory Group in project planning, decision-making, and dissemination ;
- Comparative Toxicology, using high-throughput testing methods across an Evolutionarily Diverse Model Suite of five biomedical model organisms and human cell lines to observe toxic response;
- Molecular Data Production, applying metabolomics and transcriptomics to comparative toxicology samples to trace adverse outcomes via the molecular key events preceding them ;
- Variation in Susceptibility, applying quantitative genetics and gene expression profiling to understand variation in individual susceptibility and develop empirical exposure thresholds ;
- Biomarker Discovery, PrecisionTox Data Commons, and NAM Toolbox, using machine learning to identify biomarkers for molecular key events and creating the dissemination and translation products for their use ; and
- Regulatory Analysis and Application, partnering with risk managers and regulatory agencies to identify opportunities for applying Precision Toxicology within existing regulatory structures and develop draft guidance for industry use and reporting
Towards Precision Toxicology
PrecisionTox


PrecisionTox
Grant agreement ID : 965406
Coordinated by
The University of Birmingham UK
Status
Ongoing project
Funded under
Start date
01 February 2021
End date
31 July 2026
Overall budget
€ 19 305 583,75
EU contribution
€ 19 305 583,75
“PrecisionTox aims to transform regulatory toxicology by simultaneously deploying five biomedical model species and human cell lines to uncover molecular toxicity pathways shared across the animal kingdom, enabling chemicals to be classified according to their precise effects on human health.”
“We do not rely on the strength of the science alone to engineer change.”


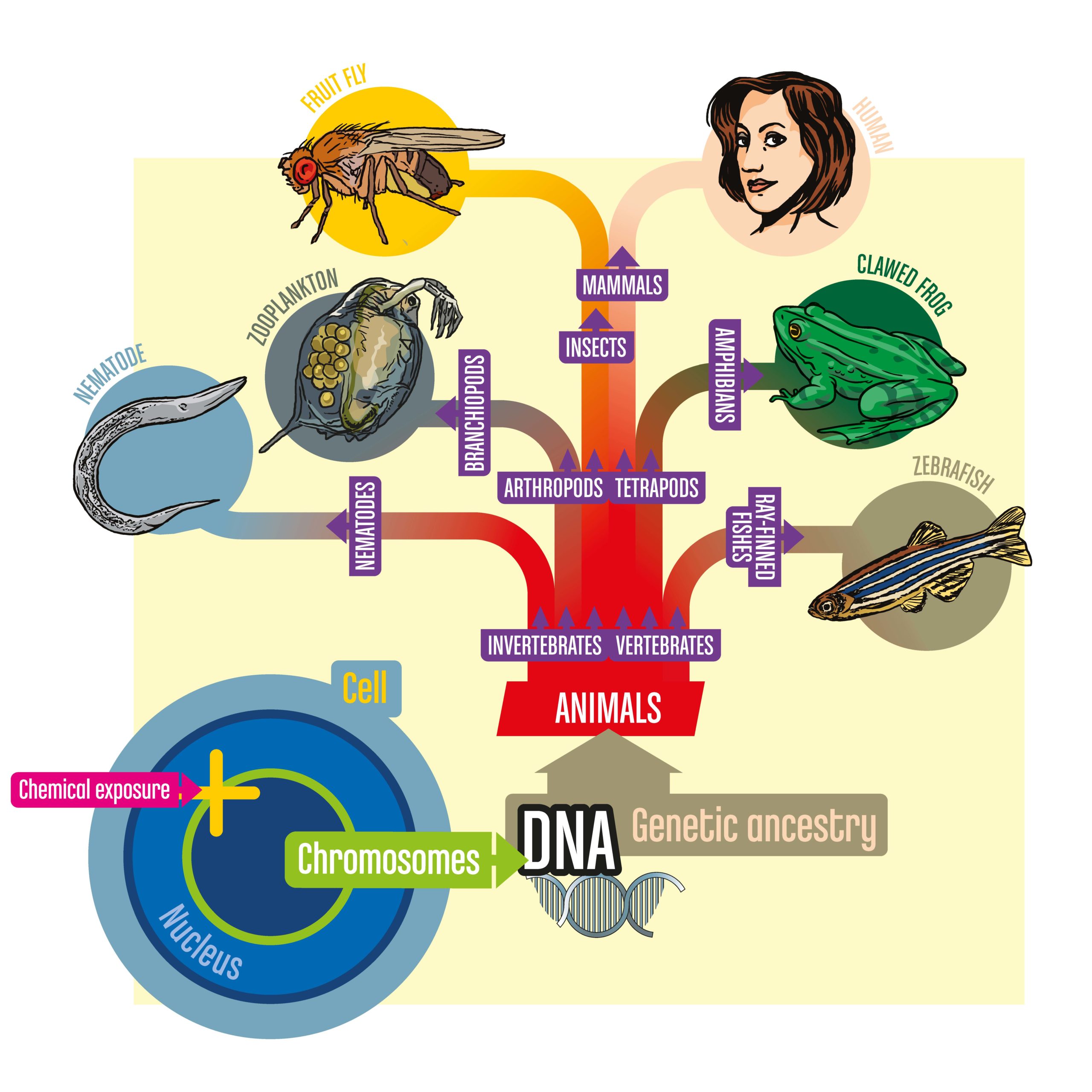
Phylogenetic Toxicology (Phylotoxicology)
Problem: Whole-organism toxicity testing is crucial but no single model is a perfect human surrogate.
Although mammals such as rats are considered the ‘gold standard’ for toxicology research, ample evidence demonstrates that these animals are not perfect predictors of human response to chemical exposure. Indeed, no animal is a perfect human substitute. This limitation — along with the desire to reduce expenses, improve the pace of testing, and reduce experimentation on animals — have driven recent toxicology efforts to instead use human-derived cell-lines. Unfortunately, focusing on cells has major drawbacks as toxic response often involves multiple cell and organ systems. The biological processes governing health cannot be fully observed by looking at isolated strains of cells.
Solution: Evolutionarily diverse model organisms plus human cell lines (Phylotoxicology).
Our consortium’s approach overcomes these limitations by leveraging the power of the phylogenetic tree, also known as the tree of life. All animals share the same genetic ancestry and, despite having branched off into diverse forms through evolution, animals continue to share much of the same DNA. Importantly, genes that govern disease response are among the most likely to be shared among different animals. As a result, instead of using traditional mammal models like rats, greater accuracy can be achieved by using a diverse range of biomedical model species — fruit flies, water fleas, round worms, and embryos of frogs and zebrafish — along with human cell lines to observe what happens when organisms are exposed to chemicals. Moreover, by using advanced approaches to analyzing activity at the molecular level, we can identify the fundamental biological mechanisms by which these organisms — and humans — respond to toxic chemical exposure.

Variation in Susceptibility
Problem: Exposure limits are set through guesswork.
Current methods for establishing safe exposure levels to toxic chemicals begin with quantities known to cause harm to one specific breed of mammal – usually a type of rat – and then apply some factor of 10 as a safety buffer. For example, if the presence of 5 parts per million of a chemical in water causes cancer in the specific rat strain used in the experiment, then the safe exposure level might be set at 0.05 parts per million for a human water supply (applying 102 as the ‘adjustment factor’). This guesswork not only risks putting people in harm’s way, but also might overestimate potential harm, leading to unnecessary anxiety and regulation. Additionally, this arbitrary approach fails to take into account that some people (and animals) will be more vulnerable to harm from a given toxic chemical than others due to genetic variation in susceptibility.
Solution: Study markers of susceptibility.
PrecisionTox addresses this challenge by conducting a detailed study of population variation with respect to the genes governing toxic response. Susceptibility to harm from exposure to a given chemical is a trait and, like any other trait, varies among individuals. By identifying gene sequences modulating molecular toxicity pathways, and by evaluating linkages between the genetic targets and individual susceptibility using unique genetically diverse model systems and human cell lines, informed estimates of safe exposure become possible.

Embedded Translation
Problem: Scientific answers are not sufficient; change requires buy-in from decision makers.
Although there is broad interest in moving away from traditional animal testing, replacing old standards with new ones is a difficult task. Change often produces anxiety, and decision makers can be reluctant to promote approaches with which they are unfamiliar.
Solution: Co-produce PrecisionTox with key decision makers.
Rather than push scientific findings towards regulators and private companies at the end of the project, PrecisionTox instead works directly with a Stakeholder Advisory Group to gain insight, guidance, and feedback on producing results that can be used in the real world. This group of experienced regulators and private sector experts actively participates in shaping project activities — including selecting chemicals for testing, identifying and addressing challenges to uptake of new testing methods, participating in case studies for integrating findings into regulation, and helping to spread the word about project results.
Ensuring Findability, Accessibility, Interoperability and Reusability of data: FAIR principles
In PrecisionTox, the results and the data will come from different sources, technologies and studies, encompassing legacy and newly produced work. To tackle this, we will enact smart data handling strategies to ensure the Findability, Accessibility, Interoperability and Reusability (FAIR) of all digital assets. Today FAIR has de facto become a global norm for good data management and has guided data policies actions and professional practices in the public and private sectors, across all disciplines. PrecisionTox put the FAIR Principles into practices to deliver actionable data, usable by humans and machines, in all phases of the data life cycle: from data generation and access, data archive and publication, to the report of data and results of regulatory significance.
To learn more about the FAIR principles, view the recording of one of our Café Scientifique webinars: FAIR data: no longer optional, but it takes a village!

















